


Send us your email to receive your password
Study Design

Management Framework
The Management Framework includes all the policies and procedures for the Biobanque québécoise de la COVID-19 (BQC19). It is available in section "Available documents".
Biological samples and data access
Access to biological specimens and to high quality data from SARS-CoV-2 positive and negative patients is essential to better understand the determinants of COVID-19. Collected biospecimens are a limited, very valuable and non renewable resource. Therefore, requests for access to these samples will be examined rigorously to maximize the sample use and to ensure that the proposed analyses are fully justified and will have a potentially important impact on the advancement of research on COVID-19. All biological samples collected will be processed in one of the BQC19 participating institutions according to the Standard Operating Procedures (SOPs) in place to ensure optimal preservation of the samples. These SOPs also require all clinical data to be electronically linked to the biospecimens in the BTRSRV2 data storage and management system, which will provide a regularly updated inventory of all specimens and data.
Data and scientific knowledge are common assets and as such must be shared within an appropriate framework. The BQC19 fully subscribes to the Wellcome Trust statement: "Sharing research data and findings relevant to the novel coronavirus (COVID-19) outbreak" disponible sur: https://wellcome.ac.uk/press-release/sharing-research-data-and-findings-relevant-novel-coronavirus-covid-19-outbreak. COVID-19 represents an important and urgent threat to global health and the BQC19 is committed to ensuring that research results derived from access to its resources and data are rapidly and openly shared to inform public health response and contribute to saving lives.
Specifically, and in alignment with the Wellcome Trust statement:
- Data will be shared with the scientific community, public health agencies and the WHO as quickly and as widely as possible, along with the protocols and standards used in collecting these data.
- All peer reviewed research publications that are relevant to the current pandemic will be immediately released in Open Access or be accessible free of charge at least for the duration of the outbreak.
Researchers who have been granted access to the BQC19 biological samples and data to conduct research will be required to provide the BQC19 a copy of all the data derived from this research. This will be specified in the Material/Data Transfer Agreements (MTAs/DTAs) that will be put in place. The exact nature of these data and the time frame to provide them will be determined during the access granting process and will be specified in the MTAs/DTAs. These data will become an integral part of the BQC19 and will be made available to future researchers accessing BQC19 data, thereby enabling them to access these rich datasets.
Researchers who will use the BQC19 biological samples and data will be required to acknowledge their source, i.e., the BQC19, in all related oral or written scientific communications. They will also be required to acknowledge the BQC19 funders, i.e., the Fonds de recherche du Québec-Santé (FRQS), Génome Québec, and the Public Health Agency of Canada. A copy of all publications and of documents referencing them, e.g., media releases, will be sent to the Manager of the BQC19. This mandatory requirement will also be included in the MTAs/DTAs.
Available Material
| Available material (currently) |
|---|
| For adults |
| RNA extract (from PAXgene tube) |
| Whole blood |
| DNA extract (from whole blood on ACD) |
| Plasma (from ACD tubes) |
| PMBC (in very limited supply) |
| Serum |
| For children |
| RNA extract (from PAXgene tube) |
| Whole blood |
| DNA extract (from whole blood on ACD) |
| Plasma (from ACD tubes) |
| PMBC (in very limited supply) |
| Serum |
| Urine (possible) |
| Feces |
| Nasal swab (possible) |
| Available material (to come) |
| For pregnant women (in very limited quantity) |
| Vaginal swabs |
| Amniotic fluid sample |
| Cord blood sample |
| Breast milk sample |
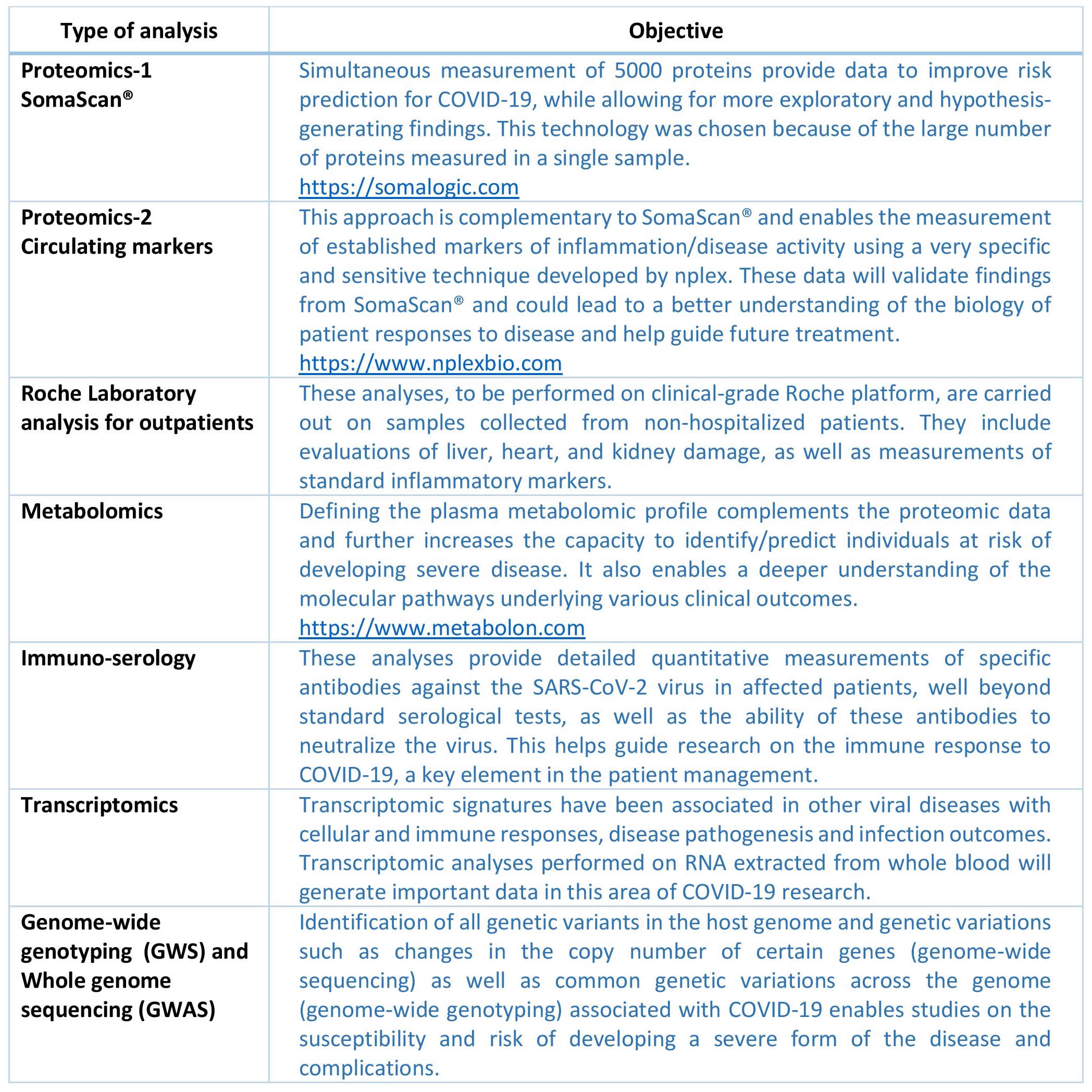
Clinical Data
| Hospitalized | Follow up post discharge/diagnostic | ||||||||||||
| Arrival | J0 | J2 | J7 | J14 | J30 | Leave | 1M | 3M | 6M | 12M | 18M | 24M | |
| Participant | |||||||||||||
| Sex at birth | • | ||||||||||||
| Country of birth | • | ||||||||||||
| Vital status | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Date of last known vital status | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Date of death | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Time of death | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Location of death | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Cause of death | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Pediatric Participant | |||||||||||||
| Infant (Less than 1 year old) | • | ||||||||||||
| Birth weight | • | ||||||||||||
| Gestational Outcome | • | ||||||||||||
| Obstetrics (if applicable) | |||||||||||||
| Patient pregnant ? | • | ||||||||||||
| Expected date of delivery | • | ||||||||||||
| Post-partum (childbirth in the last year) | • | ||||||||||||
| Pregnancy Outcome | • | ||||||||||||
| Delivery date | • | ||||||||||||
| COVID status of the baby | • | ||||||||||||
| Baby tested for mother's ARI infection | • | ||||||||||||
| Result | • | ||||||||||||
| Method | • | ||||||||||||
| Type of participant | |||||||||||||
| Type of participant | • | ||||||||||||
| Main (or primary) diagnosis related to hospitalization | • | ||||||||||||
| employed as a healthcare worker | • | ||||||||||||
| employed in a microbiology laboratory | • | ||||||||||||
| living where | • | ||||||||||||
| living with | • | ||||||||||||
| Demographics | |||||||||||||
| Age at time of visit | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Height | • | ||||||||||||
| Weight | • | ||||||||||||
| BMI | • | ||||||||||||
| Smoking and drug use | |||||||||||||
| Smoking status | • | ||||||||||||
| Electronic cigarette | • | ||||||||||||
| Comments about smoking history | • | ||||||||||||
| Drug use | • | ||||||||||||
| Type of drug | • | ||||||||||||
| Vital signs and daily assessment form (within the previous 24 hours) | |||||||||||||
| Temperature | • | • | • | • | • | • | |||||||
| Respiratory rate | • | • | • | • | • | • | |||||||
| Heart rate | • | • | • | • | • | • | |||||||
| O2 saturation at room air | • | • | • | • | • | • | |||||||
| Oxygen administered | • | • | • | • | • | • | |||||||
| O2 saturation with oxygen therapy | • | • | • | • | • | • | |||||||
| FiO2 | • | • | • | • | • | • | |||||||
| Systolic / Diastolic blood pressure | • | • | • | • | • | • | |||||||
| AVPU scale | • | • | • | • | • | ||||||||
| Glasgow score (GCS/15) | • | • | • | • | • | ||||||||
| Urine output over 24h | • | • | • | • | • | ||||||||
| Documented symptoms | |||||||||||||
| Asymptomatic | • | • | • | • | • | • | • | ||||||
| Date of earliest symptoms | • | • | • | • | • | • | • | ||||||
| Fever | • | • | • | • | • | • | • | ||||||
| Sore throat | • | • | • | • | • | • | • | ||||||
| Cough | • | • | • | • | • | • | • | ||||||
| Shortness of breath (Dyspnea) | • | • | • | • | • | • | • | ||||||
| Bloody sputum/haemoptysis | • | • | • | • | • | • | • | ||||||
| Runny nose (Rhinorrhea) | • | • | • | • | • | • | • | ||||||
| Pharyngitis | • | • | • | • | • | • | • | ||||||
| Ear pain | • | • | • | • | • | • | • | ||||||
| Joint pain (Arthralgia) | • | • | • | • | • | • | • | ||||||
| Fatigue | • | • | • | • | • | • | • | ||||||
| Chest pain | • | • | • | • | • | • | • | ||||||
| Abdominal pain | • | • | • | • | • | • | • | ||||||
| Muscle aches (Myalgia) | • | • | • | • | • | • | • | ||||||
| Wheezing | • | • | • | • | • | • | • | ||||||
| Nausea / vomiting | • | • | • | • | • | • | • | ||||||
| Headache | • | • | • | • | • | • | • | ||||||
| Confusion/altered mental status | • | • | • | • | • | • | • | ||||||
| Diarrhea | • | • | • | • | • | • | • | ||||||
| Seizures | • | • | • | • | • | • | • | ||||||
| Red eye (Conjunctivitis) | • | • | • | • | • | • | • | ||||||
| Skin rash | • | • | • | • | • | • | • | ||||||
| Anorexia | • | • | • | • | • | • | • | ||||||
| Leg swelling (edema) | • | • | • | • | • | • | • | ||||||
| Trouble speaking (aphasia/dysphagia) | • | • | • | • | • | • | • | ||||||
| Loss of taste / loss of smell | • | • | • | • | • | • | • | ||||||
| Weakness or numbness at the extremities | • | • | • | • | • | • | • | ||||||
| Past medical conditions | |||||||||||||
| Prior stroke | • | ||||||||||||
| Previous transient ischemic attack (TIA) | • | ||||||||||||
| Atrial fibrillation or flutter | • | ||||||||||||
| Other chronic neurological disorder (other than stroke/TIA) | • | ||||||||||||
| Prior myocardial infarction | • | ||||||||||||
| Coronary artery disease | • | ||||||||||||
| Heart failure | • | ||||||||||||
| Left ventricular pre-hospitalization ejection fraction | • | ||||||||||||
| Other chronic cardiac disease | • | ||||||||||||
| Chronic hematologic disease | • | ||||||||||||
| Diabetes | • | ||||||||||||
| Obesity | • | ||||||||||||
| Hypertension | • | ||||||||||||
| Asthma | • | ||||||||||||
| Rheumatologic disease | • | ||||||||||||
| Chronic kidney disease | • | ||||||||||||
| Dialysis | • | ||||||||||||
| COPD (emphysema, chronic bronchitis) | • | ||||||||||||
| Severity of COPD | • | ||||||||||||
| Pulmonary hypertension | • | ||||||||||||
| Other chronic lung disease | • | ||||||||||||
| Dementia | • | ||||||||||||
| Psychiatric disease | • | ||||||||||||
| Chronic liver disease | • | ||||||||||||
| Malnutrition | • | ||||||||||||
| Malignant neoplasm | • | ||||||||||||
| Type of cancer | • | ||||||||||||
| Metastatic | • | ||||||||||||
| Receiving Tx | • | ||||||||||||
| AIDS/HIV | • | ||||||||||||
| Imunossupressed state | • | ||||||||||||
| Other past medical cond. | • | ||||||||||||
| Home medications | |||||||||||||
| • | |||||||||||||
| ACE Inhibitor or Angiotensin receptor blocker | |||||||||||||
| Systemic steroids | • | ||||||||||||
| Anticoagulants | • | ||||||||||||
| Colchicine | • | ||||||||||||
| Other(s) immunosuppressive medications including anti-rejection Rx, and chemotherapy | • | ||||||||||||
| Laboratory results | |||||||||||||
| White blood cells count | • | • | • | • | • | max | |||||||
| Neutrophils count | • | • | • | • | • | max | |||||||
| Lymphocytes count | • | • | • | • | • | min | |||||||
| Monocytes count | • | • | • | • | • | max | |||||||
| Eosinophils count | • | • | • | • | • | max | |||||||
| Basophils count | • | • | • | • | • | max | |||||||
| Platelet count | • | • | • | • | • | min | |||||||
| Hemoglobin measurement | • | • | • | • | • | min | |||||||
| Urea | • | • | • | • | • | max | |||||||
| Creatinine | • | • | • | • | • | max | |||||||
| NT-proBNP | • | • | • | • | • | max | |||||||
| BNP | • | • | • | • | • | max | |||||||
| Sodium Na+ | • | • | • | • | • | max | |||||||
| Potassium K+ | • | • | • | • | • | max | |||||||
| C-reactive protein (CRP) | • | • | • | • | • | max | |||||||
| Lactate dehydrogenase (LDH or LD) | • | • | • | • | • | max | |||||||
| Creatine Phosphokinase (CPK) | • | • | • | • | • | max | |||||||
| Albumin | • | • | • | • | • | min | |||||||
| AST | • | • | • | • | • | max | |||||||
| ALT | • | • | • | • | • | max | |||||||
| Procalcitonine (PCT) | • | • | • | • | • | max | |||||||
| Troponine T hs (high sensitivity) | • | • | • | • | • | max | |||||||
| Troponine I hs (high sensitivity) | • | • | • | • | • | max | |||||||
| Troponine T | • | • | • | • | • | max | |||||||
| Troponine I | • | • | • | • | • | max | |||||||
| APTT | • | • | • | • | • | max | |||||||
| International Normalized Ratio (INR) | • | • | • | • | • | max | |||||||
| Triglycerides | • | • | • | • | • | max | |||||||
| Total Bilirubin | • | • | • | • | • | max | |||||||
| Direct bilirubin (conjugated) | • | • | • | • | • | max | |||||||
| Glucose | • | • | • | • | • | max | |||||||
| Venous lactate | • | • | • | • | • | max | |||||||
| D-Dimer | • | • | • | • | • | max | |||||||
| Fibrinogen | • | • | • | • | • | max | |||||||
| Ferritin | • | • | • | • | • | max | |||||||
| IL-6 | • | • | • | • | • | max | |||||||
| CD4 | • | • | • | • | • | min | |||||||
| CD8 | • | • | • | • | • | min | |||||||
| Support | |||||||||||||
| Ventilatory support | • | • | • | • | • | • | |||||||
| Oxygen therapy with cannula/mask | • | • | • | • | • | • | |||||||
| High-flow nasal cannula (HFNC) | • | • | • | • | • | • | |||||||
| Non-invasive ventilation CPAP/BPAP | • | • | • | • | • | • | |||||||
| Invasive with mecanical ventilation | • | • | • | • | • | • | |||||||
| Number of days | • | ||||||||||||
| SpO2 (the lowest associated ith the highest support) | • | • | • | • | • | • | |||||||
| FiO2 (associated with the previous SPO2) | • | • | • | • | • | • | |||||||
| Adjuvant therapy | • | • | • | • | • | • | |||||||
| Vasopressor / inotropic support | • | • | • | • | • | • | |||||||
| Number of days | • | ||||||||||||
| Prone positioning | • | • | • | • | • | • | |||||||
| iNO | • | • | • | • | • | • | |||||||
| ECMO | • | • | • | • | • | • | |||||||
| HFOV | • | • | • | • | • | • | |||||||
| Tracheostomy | • | • | • | • | • | • | |||||||
| Blood transfusion | • | • | • | • | • | • | |||||||
| Neuromuscular blocking agents | • | • | • | • | • | • | |||||||
| Dialysis / hemofiltration | • | • | • | • | • | • | |||||||
| Other(s), specify | • | • | • | • | • | • | |||||||
| Frailty | |||||||||||||
| Clinical Frailty Scale | •* | • | • | • | • | • | • | • | |||||
| Hospitalization summary | |||||||||||||
| Date and time of arrival at this hospital | • | ||||||||||||
| Emergency visit only | • | ||||||||||||
| Is it a transfer from another establishment? | • | ||||||||||||
| Facility name | • | ||||||||||||
| If transferred from other facility, date of initial admission | • | ||||||||||||
| Date of ICU admission (if applicable) | • | ||||||||||||
| Date of ICU discharge (if applicable) | • | ||||||||||||
| Date of hospital discharge | • | ||||||||||||
| Disposition | • | ||||||||||||
| Discharge status | • | ||||||||||||
| If COVID positive, what is the most severe degree of severity reached? (according to the WHO) | • | ||||||||||||
| Ability to self-care at discharge vs pre-COVID | • | ||||||||||||
| Level of care (last status) | • | ||||||||||||
| COVID diagnosis and pathogen test | |||||||||||||
| Date of diagnostic test for COVID19 | • | ||||||||||||
| Result | • | ||||||||||||
| Type of sampling | • | ||||||||||||
| Documented positive viral infections during hospitalization | • | ||||||||||||
| Adenovirus | • | ||||||||||||
| Influenza | • | ||||||||||||
| RSV | • | ||||||||||||
| Parainfluenza | • | ||||||||||||
| Rhinovirus / Enterovirus | • | ||||||||||||
| Metapneumovirus | • | ||||||||||||
| Other, specify | • | ||||||||||||
| Documented positive bacterial cultures during hospitalization | • | ||||||||||||
| Complications at any time during hospitalization | |||||||||||||
| ARDS | • | ||||||||||||
| Specify severity | • | ||||||||||||
| Bacterial pneumonia | • | ||||||||||||
| Viral pneumonia | • | ||||||||||||
| Bronchiolitis | • | ||||||||||||
| Cryptogenic organizing pneumonia (COP) | • | ||||||||||||
| Pulmonary embolism (PE) | • | ||||||||||||
| Deep vein thrombosis (DVT) | • | ||||||||||||
| Pneumothorax | • | ||||||||||||
| Pleural effusion | • | ||||||||||||
| Left ventricular function | • | ||||||||||||
| LVEF | • | ||||||||||||
| Decompensated heart failure | • | ||||||||||||
| Myocarditis | • | ||||||||||||
| Endocarditis | • | ||||||||||||
| Pericarditis | • | ||||||||||||
| New atrial fibrillation or flutter (AF) | • | ||||||||||||
| Ventricular tachycardia or fibrillation (VT/VF) | • | ||||||||||||
| Other type of cardiac arrythmia | • | ||||||||||||
| Specify | • | ||||||||||||
| Cardiac arrest | • | ||||||||||||
| Cardiac ischaemia STEMI | • | ||||||||||||
| Cardiac ischaemia NSTEMI | • | ||||||||||||
| Stroke / Cerebrovascular accident | • | ||||||||||||
| Pancreatitis | • | ||||||||||||
| Transient ischemic attack (TIA) | • | ||||||||||||
| Seizure | • | ||||||||||||
| Meningitis / Encephalitis | • | ||||||||||||
| Rhabdomyolysis / Myositis | • | ||||||||||||
| • | |||||||||||||
| Coagulation disorder / Disseminated Intravascular Coagulation | |||||||||||||
| Bacteremia | • | ||||||||||||
| Anemia | • | ||||||||||||
| Gastrointestinal haemorrhage | • | ||||||||||||
| Liver dysfunction | • | ||||||||||||
| Acute renal injury/ Acute renal failure | • | ||||||||||||
| Hyperglycemia | • | ||||||||||||
| Hypoglycemia | • | ||||||||||||
| Other | • | ||||||||||||
| Specify | • | ||||||||||||
| Other tests performed during hospital stay | |||||||||||||
| Chest X-Ray | • | ||||||||||||
| CT Thorax | • | ||||||||||||
| CT Abdomen | • | ||||||||||||
| CT Head | • | ||||||||||||
| ECG | • | ||||||||||||
| Point of care ultrasound (POCUS) | • | ||||||||||||
| Echocardiogram | • | ||||||||||||
| Echocardiography | |||||||||||||
| Coronary angiography (Cardiac catheterization) | • | ||||||||||||
| Percutaneous coronary vintervention (stented) | • | ||||||||||||
| Other imaging tests | • | ||||||||||||
| Medication (at any time during hospitalisation ) | |||||||||||||
| Remdesivir | • | ||||||||||||
| Ribavirin | • | ||||||||||||
| Lopinavir/Ritonavir ("Kaletra") | • | ||||||||||||
| Interferon alpha | • | ||||||||||||
| Interferon beta | • | ||||||||||||
| Neuraminidase inh | • | ||||||||||||
| Other antiviral | • | ||||||||||||
| Specify | • | ||||||||||||
| Azithromycin ("Zithromax") | • | ||||||||||||
| Other antibiotic | • | ||||||||||||
| Antifungal | • | ||||||||||||
| Systematic corticosteroid | • | ||||||||||||
| Hydroxychloroquine ("Plaquenil") | • | ||||||||||||
| Chloroquine ("Aralen") | • | ||||||||||||
| Ivermectin ("Stromectol") | • | ||||||||||||
| Tocilizumab ("Actemra") | • | ||||||||||||
| Sarilumab ("Kevzara") | • | ||||||||||||
| Kineret ("Anakinra") | • | ||||||||||||
| Other immunomudulator | • | ||||||||||||
| Colchicine | • | ||||||||||||
| Plasma | • | ||||||||||||
| Stem cells | • | ||||||||||||
| IVIG | • | ||||||||||||
| IVIG | • | ||||||||||||
| Follow up | |||||||||||||
| Have you been re-hospitalized since your initial visit for COVID? | • | • | • | • | • | • | |||||||
| Date of admission | • | • | • | • | • | • | |||||||
| Facility | • | • | • | • | • | • | |||||||
| Cause | • | • | • | • | • | • | |||||||
| Functional status | |||||||||||||
| Mobility | • | • | • | • | • | • | |||||||
| Self-care | • | • | • | • | • | • | |||||||
| Usual activities (i.e. work, study, housework, family or leisure activities) | • | • | • | • | • | • | |||||||
| Pain and discomfort | • | • | • | • | • | • | |||||||
| Anxiety and depression | • | • | • | • | • | • | |||||||
| Breathlessness | • | • | • | • | • | • | |||||||
| Health status | • | • | • | • | • | • | |||||||
| How much difficulty do you have | |||||||||||||
| lifting or carrying 10 lbs? | • | • | • | • | • | • | |||||||
| walking across a room? | • | • | • | • | • | • | |||||||
| transferring from a chair to a bed? | • | • | • | • | • | • | |||||||
| climbing a flight of 10 stairs? | • | • | • | • | • | • | |||||||
| fallen in the past year? | • | • | • | • | • | • | |||||||

